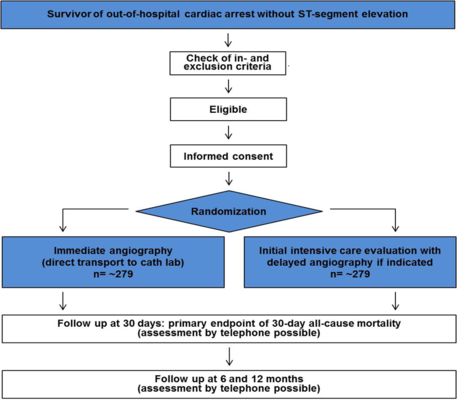
TOMAHAWK is a prospective, randomized, multi-center, controlled trial. A total of 558 patients will be randomized. The primary objective is to compare immediate coronary angiography with deferred or selective coronary angiography in survivors of out-of-hospital cardiac arrest (OHCA) without ST-segment elevation with regards to 30-day mortality.
Inclusion criteria
- Documented resuscitated out-of-hospital cardiac arrest of possible cardiac origin and return of spontaneous circulation
- Age ≥30 years
- Informed consent
Exclusion criteria
- ST-segment elevation or left bundle branch block
- No return of spontaneous circulation upon hospital admission
- Severe hemodynamic or electrical instability requiring immediate angiography/intervention (delay clinically not acceptable)
- Obvious extracardiac etiology such as traumatic brain injury, primary metabolic or electrolyte disorders, intoxication, overt hemorrhage, respiratory failure due to known lung disease, suffocation, drowning
- In-hospital cardiac arrest
- Known or likely pregnancy
- Participation in another intervention study
Patient enrollment started in November 2016.
The results of the TOMAHAWK trial will improve the treatment algorithm of patients with OHCA without ST-segment elevation. Using data from a prospective randomized trial physicians will be able to clearly decide on the approach which may increase the survival of those patients based on scientific evidence.